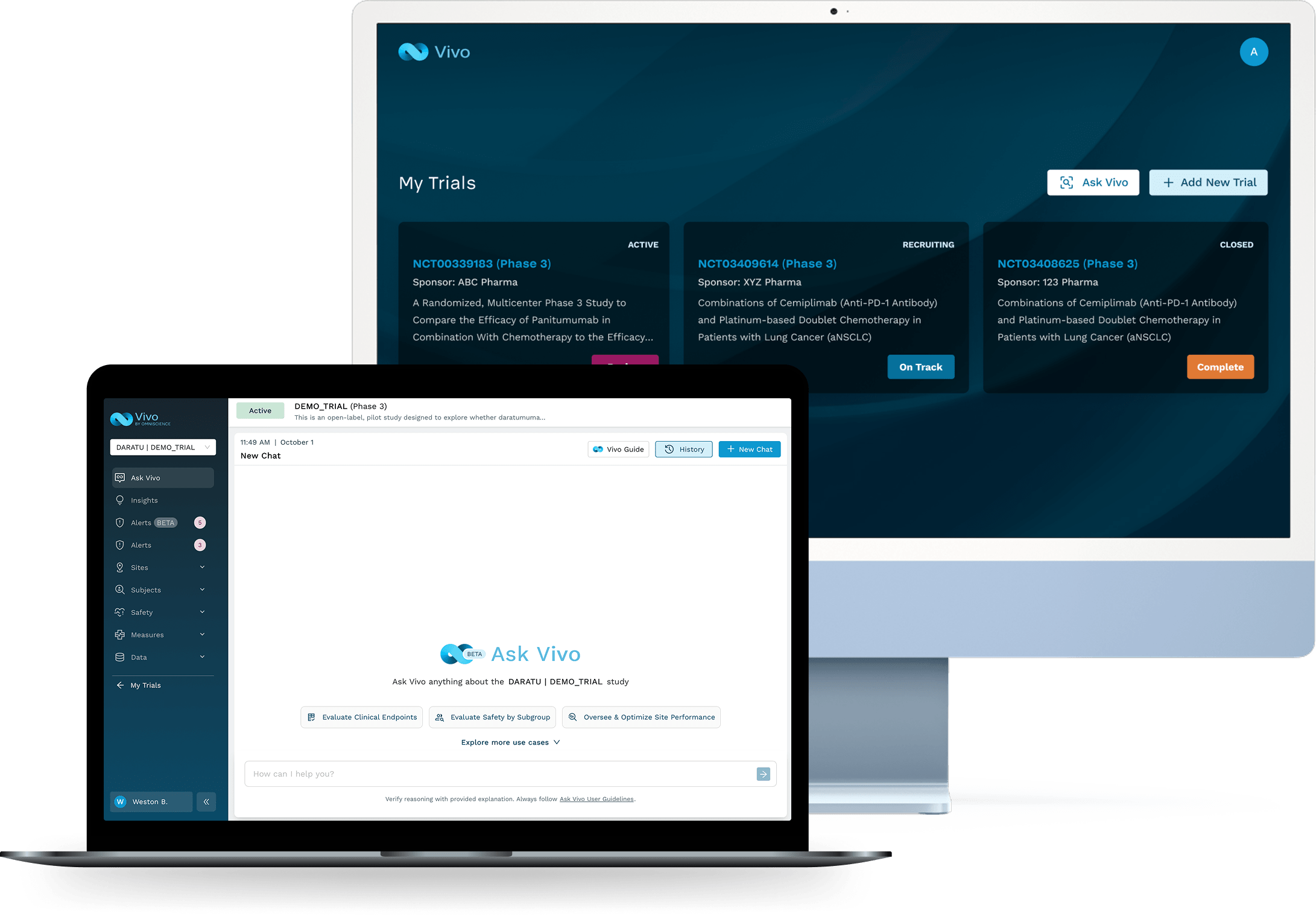
Real-Time Portfolio Intelligence for Clinical Development Leaders. Clinical development leaders can’t afford to wait weeks for reports. Vivo empowers you to stay ahead of enrollment delays, site underperformance, and emerging safety concerns as they happen. With a unified portfolio view, Vivo makes it easy to prioritize action, accelerate timelines, and walk into executive reviews fully prepared.
Download our Clinical Development One-Pager to learn how Vivo transforms fragmented trial data into portfolio-wide intelligence.
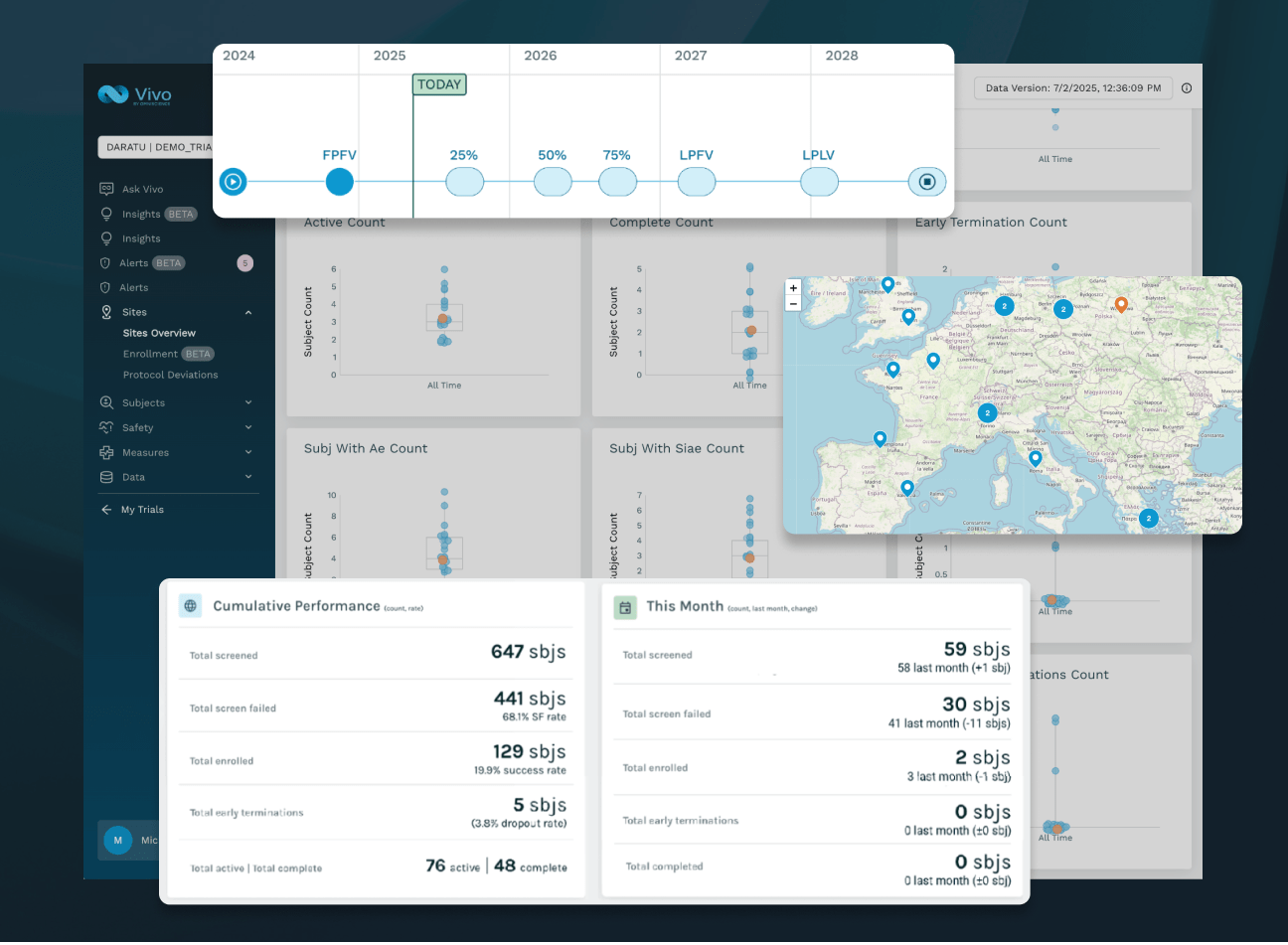
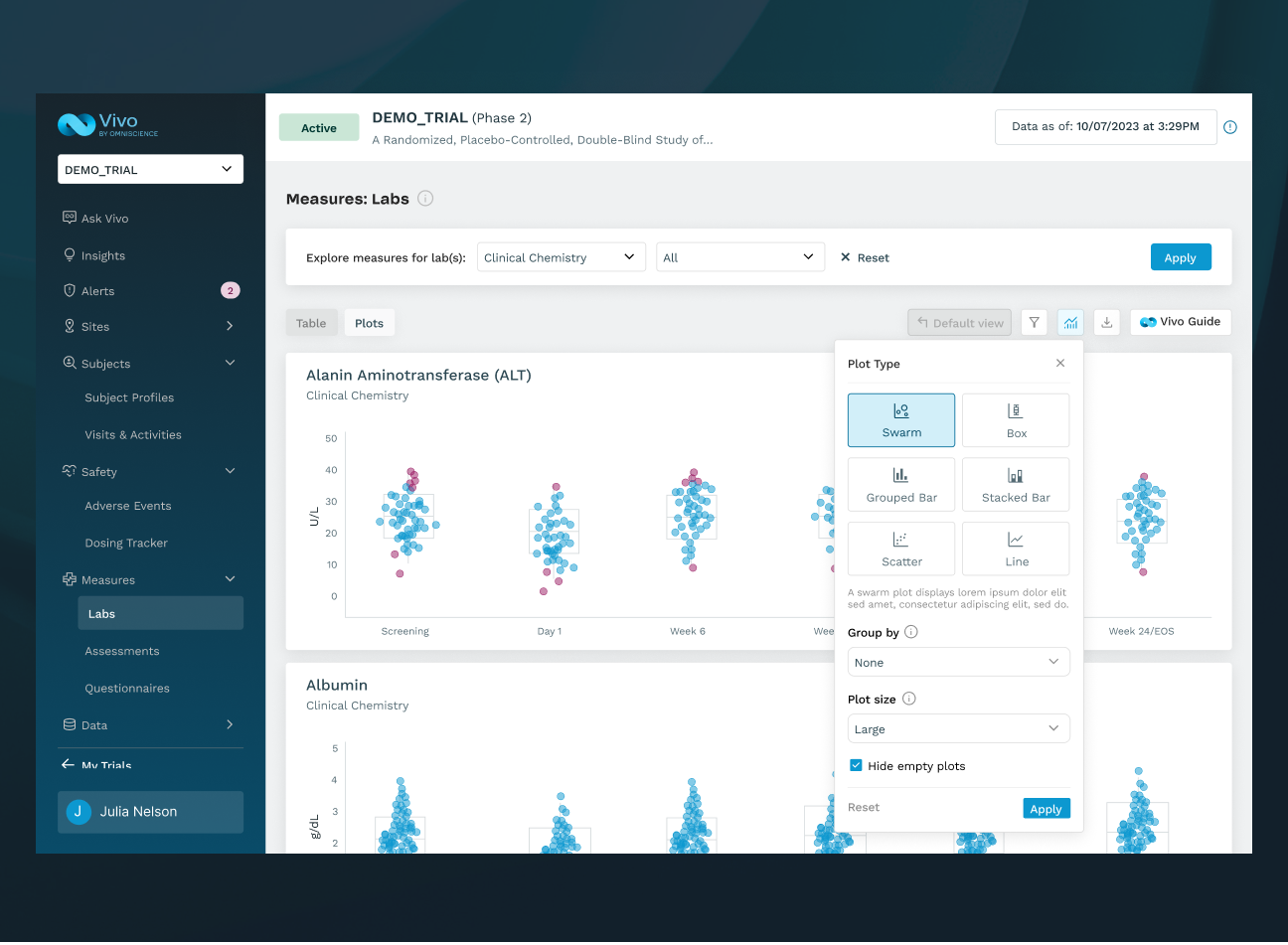
UNIFY DATA
Automatically unify clinical trial data across the portfolio to improve oversight of enrollment, retention, safety, compliance, and data integrity.
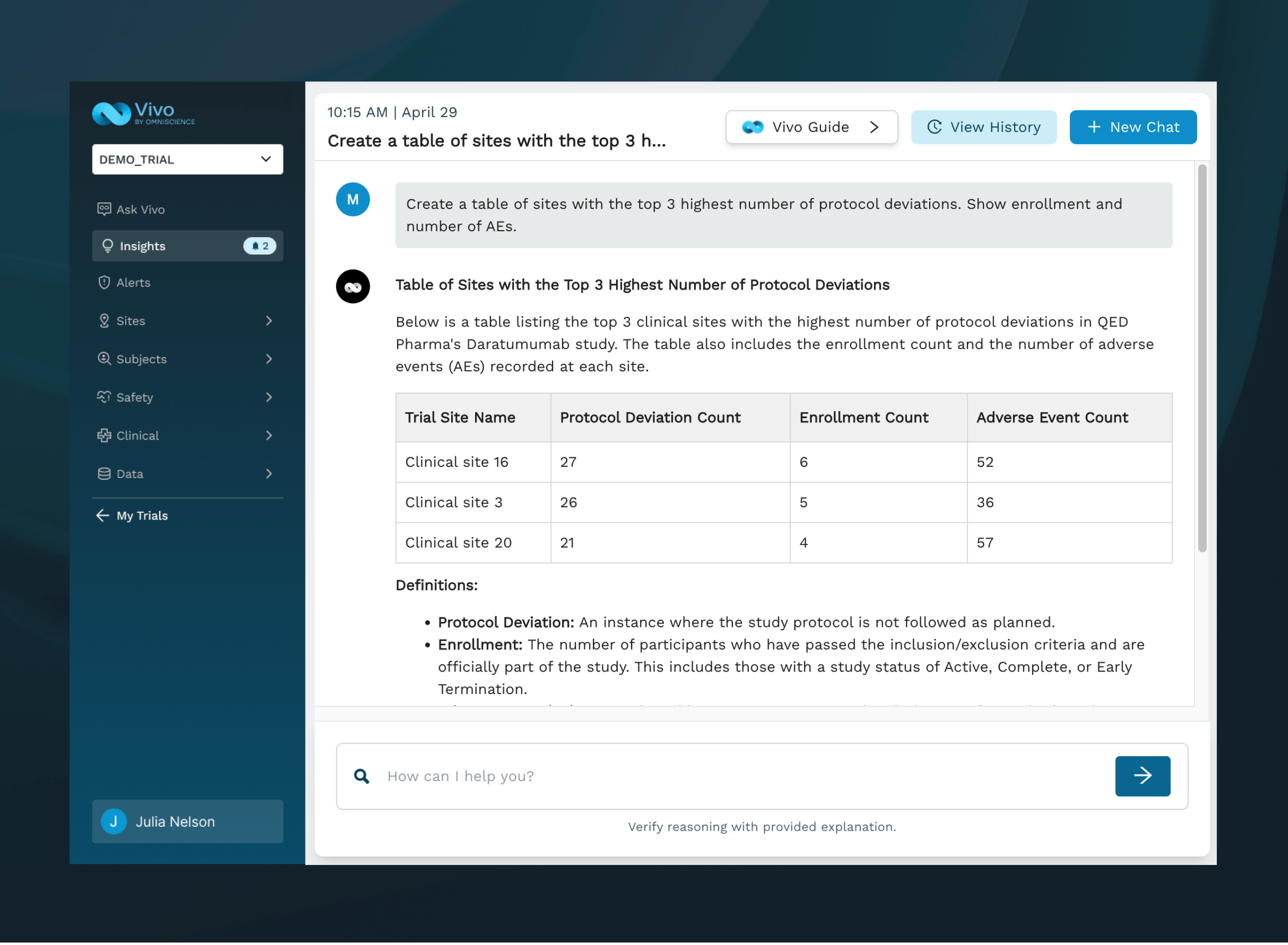
GET INSTANT ANSWERS
Talk to your clinical trial data and receive explainable answers to inform decisions and improve outcomes, all on demand.
ACT FASTER
Inform actions with role-specific recommendations to accelerate timelines and optimize trial success.